Health Insurance
Half 3 – Healthcare Economist
See my earlier posts on IRA worth negotiation on drug choice (Half 1) and producer knowledge submission (Half 2).
At this time we’ll speak in regards to the negotiation course of and the way CMS will set the utmost truthful worth (MFP)
How will CMS worth throughout dosages?
“CMS will base the only worth on the price of the chosen
drug per 30-day equal provide (slightly than per unit—resembling pill,
capsule, injection—or per quantity or weight-based metric), weighted throughout
dosage varieties and strengths.”
Is there a most worth or “ceiling” for the utmost
truthful worth (MFP) that CMS will supply?
The utmost MFP quantity shall be no increased than:
- An quantity equal to the sum of the plan-specific
enrollment weighted quantities - The decrease of: the typical non-FAMP in 2021
elevated by inflation (CPI-U) or the typical non-FAMP worth in February 2025
CMS will combination the 60 quantities decided for every NDC-11 for the chosen drug to calculate a single quantity – individually for every methodology – throughout dosage varieties, strengths, and bundle sizes of the chosen drug. These quantities can then be instantly in contrast, and the ceiling for the only MFP of the chosen drug (together with all dosage varieties and strengths) would be the decrease quantity.
Pattern packages, NDCs from secondary producers, NDCs
with no amount disbursed or NDCs with gross coated prescription drug prices of
$0 is not going to be included within the MFP calculation.
Can some claims be excluded from the MFP refund?
As soon as the MFP worth is decided, there are some instances the place
a producer wouldn’t should pay the MFP refund. These embrace:
“…[justification] codes for the drug being prospectively bought at or beneath the MFP, the producer and meting out entity having a individually negotiated refund quantity distinct from the Commonplace Default Refund Quantity, and the declare being excluded from MFP refunds underneath part 1193(d)(1) of the Act”
CMS has to justify the MFP to producers. How will it do that?
The CMS justification will comply with a 4-step course of:
- Identification of therapeutic different(s), if any, for the chosen drug. This contains FDA-approved medicine for the related indication and off-label use if included in nationally acknowledged, evidence-based tips and in a CMS-recognized compendia. CMS will start by figuring out therapeutic options inside the identical pharmacologic class as the chosen drug based mostly on properties resembling chemical class, therapeutic class, or mechanism of motion, after which additionally contemplate therapeutic options in numerous pharmacologic lessons based mostly on CMS’ evaluation of related knowledge (see query beneath).
- Measure the worth of the therapeutic options. For Half D medicine, that is whole gross coated drug price (TGCDC) internet of DIR and CGDP funds and/or the Common Gross sales Value (ASP) for Half B medicine (or prior 12 months MFP if relevant)
- Decide if drug has distinctive profit. Consider whether or not the chosen drug—relative to therapeutic options—addresses an unmet want, has a useful affect on IRA particular populations, and the extent to which the chosen drug represents a therapeutic advance in comparison with therapeutic different(s)
- Additional adjustment of preliminary worth. These changes shall be based mostly on producer submitted knowledge together with: (1) R&D prices and R&D prices recouped, (2) present unit prices of manufacturing and distribution; (3) prior Federal monetary help for novel therapeutic discovery and growth; (4) pending and authorized patent functions or exclusivities; and (5) market knowledge and income and gross sales quantity knowledge for the drug within the US., and (6) non-obligatory producer submitted knowledge.
What knowledge does CMS use to find out therapeutic options?
“…CMS will use knowledge submitted by the Major Producer and the general public, FDA-approved indications, drug classification techniques generally used within the public and business sector for formulary growth, CMS-recognized Half D compendia, broadly accepted scientific tips, the CMS led literature evaluation, drug or drug class evaluations, and peer-reviewed research.”
How may CMS set the preliminary worth supply?
The first method CMS will set it’s preliminary worth supply for
2027 is predicated on the web worth of therapeutic options.
Nevertheless…
If the chosen drug has no therapeutic different, if the costs of all therapeutic options recognized are above the statutory ceiling for the MFP…or if there’s a single therapeutic different for the chosen drug and its worth is above the statutory ceiling for the MFP, then CMS will decide the place to begin for the preliminary supply based mostly on the FSS or…“Massive 4 worth”…whichever is decrease. If the FSS and Massive 4 costs are above the statutory ceiling, then CMS will use the statutory ceiling as the place to begin for the preliminary supply.
Why did CMS select to set it’s preliminary worth based mostly on the
worth of therapeutic options?
Be aware that CMS did contemplate a wide range of choices for setting
the preliminary worth supply together with internet costs, unit price of manufacturing/distribution,
home references worth to the Federal Provide Schedule (FSS) worth, a “truthful
revenue” worth based mostly on whether or not R&D prices have been recouped and margin on
unit price of manufacturing and distribution, however settled on the web worth of
therapeutic options.
Nevertheless, it argues that the web worth of therapeutic options—regardless of
limitations—is a most popular choice:
“In taking this method, CMS acknowledges that the therapeutic different(s) for a specific drug might not be priced to replicate its scientific profit, nevertheless, utilizing Web Half D Plan Cost and Beneficiary Legal responsibility, ASPs, or MFPs of therapeutic options allows CMS to begin growing the preliminary supply inside the context of the associated fee and scientific good thing about a number of medicine that deal with the identical illness or situation. By utilizing the worth(s) of the chosen drug’s therapeutic different(s), CMS will be capable of focus the preliminary supply on part 1194(e)(2) components by adjusting this place to begin relative as to if the chosen drug provides extra, much less, or related profit in comparison with its therapeutic different(s).”
What components will affect CMS’s determination to regulate its
preliminary supply?
Some issues embrace:
- Medical profit conferred by the chosen drug
in comparison with its therapeutic different(s), - Influence on patient-reported outcomes and affected person
expertise - Influence on caregivers
- Utilization patterns of the chosen drug versus its
therapeutic different(s) - Suggestions from consultations with clinicians,
sufferers or affected person organizations, tutorial specialists, and/or the FDA - Influence on CMS particular populations (people
with disabilities, the aged, people who’re terminally unwell, youngsters,
and different Medicare beneficiaries) - Whether or not or not the therapy meets an unmet
medical want
Key related info that shall be thought-about embrace: “…peer-reviewed
analysis, skilled reviews or whitepapers, clinician experience, real-world
proof, and affected person expertise.” Key
outcomes of curiosity to be thought-about embrace a wide range of outcomes, together with
patient-centered outcomes, and affected person expertise.
Though CMS notes that it’ll not use cost-effectiveness
evaluation based mostly on QALYs, it has not dominated on whether or not it could possibly use different
approaches resembling equal worth of life years gained (evLYG), well being years in
whole (HYT) or generalized and risk-adjusted QALYs (GRA-QALYs).
These components will affect the worth via a qualitative determination
course of.
Will caregiver expertise affect CMS choices?
Sure. The
steerage says that “CMS may additionally contemplate the caregiver perspective to the
extent that it displays instantly upon the expertise or related outcomes of
the affected person taking the chosen drug.”
Does CMS contemplate price when evaluating if a therapy is
a therapeutic advance?
Sure.
“CMS will decide the extent to which a specific drug represents a therapeutic advance as in comparison with its therapeutic different(s) by inspecting enhancements in outcomes in comparison with its therapeutic different(s) (e.g., chosen drug is healing versus a therapeutic different that delays development) and can contemplate the prices of such therapeutic different(s). CMS might contemplate a specific drug to symbolize a therapeutic advance if proof signifies that the chosen drug represents a considerable enchancment in outcomes in comparison with the chosen drug’s therapeutic different(s) for a sign(s).”
How will the negotiation course of work?
That is summarized within the graphic beneath.
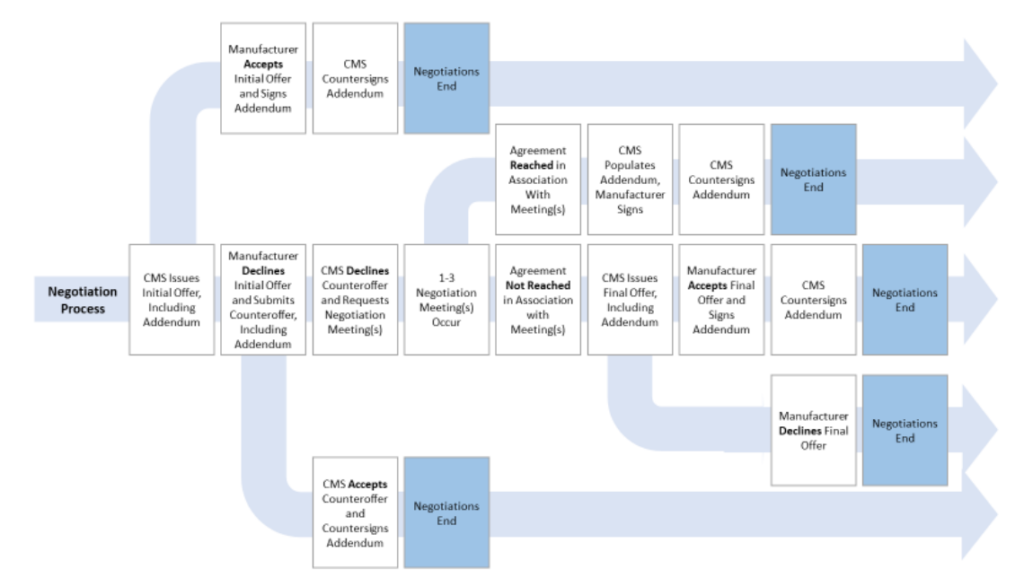
Extra element will be discovered within the CMS steerage doc right here.
Related Posts
- An outline – Healthcare Economist
A paper by Kogut (2024) has a pleasant overview of the organizations that develop pharmacy…
- A primer on formularies – Healthcare Economist
Hydery and Reddy (2024) present a pleasant primer on drug…
- evLYG Defined – Healthcare Economist
One concern when utilizing high quality adjusted life years (QALY) to measure well being features…













